- Details
- By Native News Online Staff
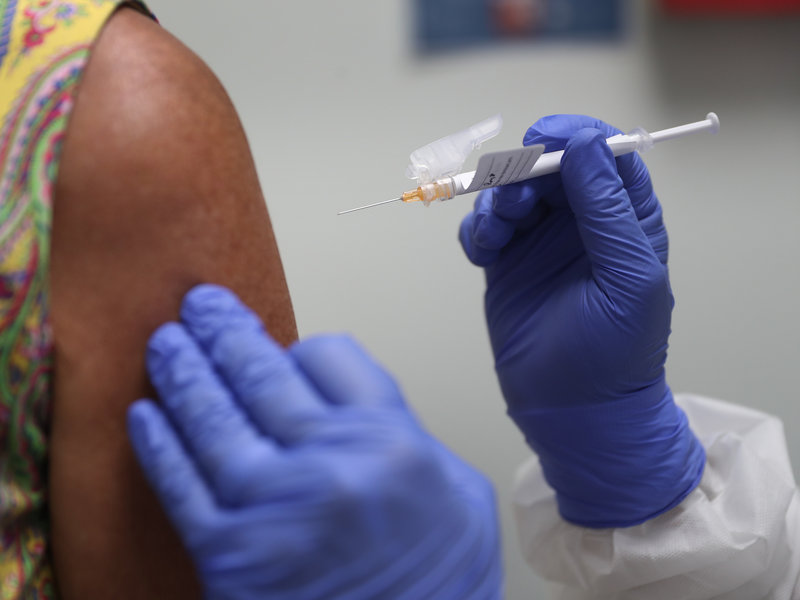
BELLINGHAM, Wash. — The Lummi Nation, which made headlines in September for being among the first tribes nationwide to participate in coronavirus vaccination trials, has pulled out of the AstraZeneca Covid-19 vaccine trial. The announcement was made by the Lummi Indian Business Council.
Lummi Nation doctors noted ongoing communication problems with AstraZeneca representatives as a primary factor in the tribe’s decision to withdraw from the trial, according to a press release. The British multinational drug company has also placed the trial on hold while it “assesses adverse reactions experienced by some trial volunteers.”
“We will continue to look for ways to protect our people from this virus,” said Lawrence Solomon, Chairman of the Lummi Indian Business Council, in a press release. “But after consultation with the Lummi Public Health Department, it was clear that the AstraZeneca vaccine trial was not a good fit.”
The tribe has not ruled out the possibility of participating in a vaccination trial. The Lummi Public Health Department has partnered with the University of Washington Department of Medicine and U.S. National Institutes of Health Coronavirus Prevention Network to explore further Covid-19 vaccine trials that will require volunteer participants.
Lummi Nation was one of three tribes in the country to enroll in a coronavirus vaccine trial, which included the Navajo Nation, the largest reservation in the United States. The decision to participate in the trials drew criticism and concern from tribal members from both tribes, who feared they were being used as guinea pigs.
“Native peoples are at greater risk for severe symptoms and death from Covid-19,” said Dr. Dakotah Lane, medical director of the Lummi Nation and Lummi tribal member, in a press release. “Yet we are rarely participants in the testing of life-saving vaccines and medications. This is a significant disadvantage to determining whether a vaccine is effective for American Indian populations.”
According to the CDC, Native Americans test positive, are hospitalized, and die from Covid-19 at disproportionately higher rates than non-Native populations.
“We expect any vaccine trial we enroll in to meet the highest standards,” said Lane. “While the AstraZeneca trial is not a good fit at this time, we will assess future trials to see if they are safe and appropriate for our tribal members who wish to participate.”
In a press release, Solomon said the Lummi Nation has seen the horrible impact of Covid-19 in other Native communities. “We grieve with our relatives across the country for the impact this virus will have on future generations of our Native peoples,” he added. “Our greatest responsibility will always be the health and well-being of our community. Just as we have since the early days of the virus outbreak, we will continue to look at every available preventive measure to stop the spread in our community.”
More Stories Like This
New Mexico Will Investigate Forced Sterilization of Native American WomenUSDA Expands Aid for Lost Farming Revenue Due to 2025 Policies
Two Feathers Native American Family Services Wins 2026 Irvine Leadership Award
Bill Would Give Federal Marshals Authority to Help Tribes Find Missing Children
Indian Health Service to Phase Out Mercury-Containing Dental Amalgam by 2027