- Details
- By Native News Online Staff
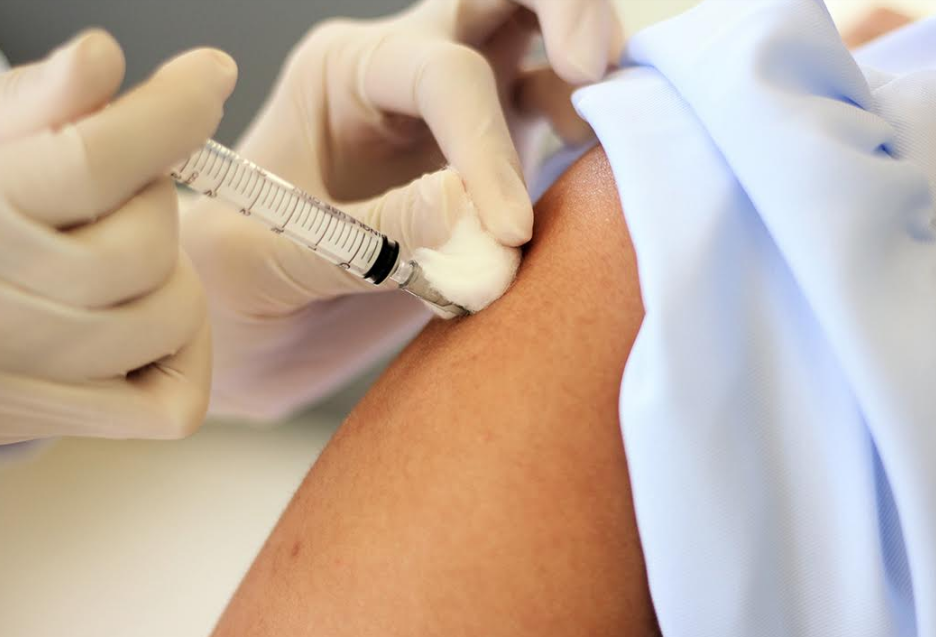
BELLINGHAM, Wash. — The Lummi Public Health Department has submitted an application to participate in a Novavax Covid-19 vaccine trial, a move that is the first step in determining whether the tribe will enroll in the trial.
Earlier this month, the tribe withdrew from the AstraZeneca Covid-19 vaccine trial, with Lummi Public Health citing communication problems with AstraZeneca representatives following a pause in the study due to adverse reactions experienced by trial participants in the United Kingdom.
"Native peoples are at higher risk of severe symptoms or death from the coronavirus," said Dakotah Lane, medical director of the Lummi Public Health Department and member of the Lummi Nation, in a statement released by the Lummi Nation. "The AstraZeneca trial was not a good fit, but we continue to believe it's important for our people to have the opportunity to volunteer for a trial as we're at a much higher risk than other populations."
There will be a three-part process to determining whether the Lummi Nation will participate in the vaccine trial, including review and recommendations by the Northwest Indian College Institutional Review Board (IRB), the Lummi Health and Family Services Commission, and the Lummi Indian Business Council (LIBC). A recommendation will then be forwarded to the Lummi Tribal Health Commission, a six-member group made up of Lummi tribal members, which will review the application and respond to any public concerns.
If the health commission decides to recommend the vaccine trial, the decision to particpate will then be put to a vote by the LIBC. If approved, the Lummi Public Health Department could begin registering volunteers sometime in November.
"We know there are concerns from our tribal members and we have taken this to heart," said Lawrence Solomon, chairman of the Lummi Nation, in a statement. "This decision will be made carefully and with community input. Other tribes have experienced such loss from this virus that we want to provide as many options to protect people as possible."
In a statement, Lane emphasized how much is at stake for Native communities when it comes to the impact of Covid-19.
"If the experts and community approve of proceeding with the Novavax vaccine trial, this will be just one more available option for our tribal members who want to participate."
More Stories Like This
New Mexico Will Investigate Forced Sterilization of Native American WomenUSDA Expands Aid for Lost Farming Revenue Due to 2025 Policies
Two Feathers Native American Family Services Wins 2026 Irvine Leadership Award
Bill Would Give Federal Marshals Authority to Help Tribes Find Missing Children
Indian Health Service to Phase Out Mercury-Containing Dental Amalgam by 2027