- Details
- By Native News Online Staff
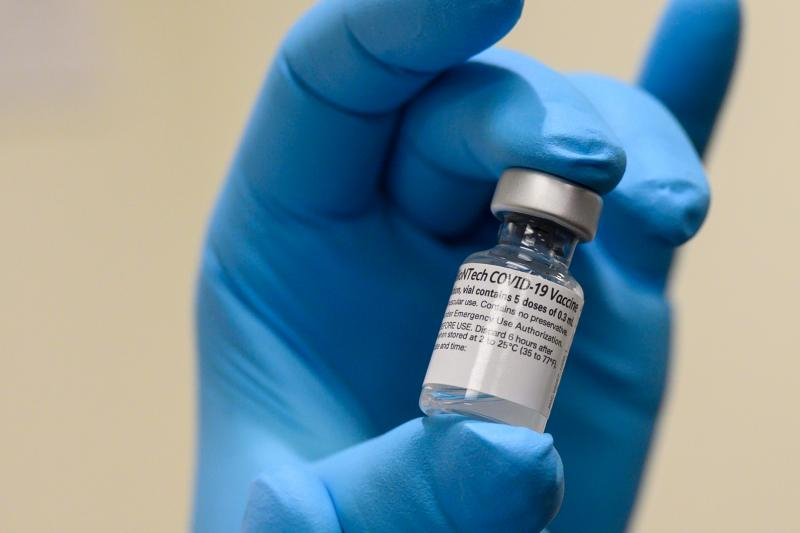
WASHINGTON — The Food and Drug Administration (FDA) on Monday said 12 to 15-year-old children are now eligible to receive the Pfizer-BioNTech Covid-19 vaccine.
Indian Health Service (IHS) will await guidance from the Advisory Council on Immunization Practices (ACIP) and adoption by the Centers for Disease and Controls (CDC) before issuing its approval to IHS facilities across Indian Country. The ACIP will meet tomorrow on Wednesday, May 12 and then make an appropriate use of the Pfizer vaccine to the adolescent population, according to a statement sent to Native News Online on Tuesday morning.
While IHS has not made a final determination about administering the vaccine to this age group, Dr. L. Christensen, acting chief medical officer, at Indian Health Service, sees the benefits of the vaccine given to 12 – 15 years old children.
“So many young people have missed out on important moments in their life over the past year – sports seasons, proms and school functions, seeing grandparents, or even just hanging out with their friends. Getting vaccinated can help return our kids to their normal lives. This is a significant step in the fight against the Covid-19 pandemic,” Christensen said.
"Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations,” Dr. Janet Woodcock, the acting FDA commissioner said.
She said the expansion to include this age group brings us closer to returning to a sense of normalcy. The timing of yesterday’s approval allows for many middle and high school students to be fully vaccinated by the time school resumes after summer recess.
According to the Centers for Disease Control and Prevention (CDC), from March March 1, 2020 through April 30, 2021, approximately 1.5 million Covid-19 cases in individuals 11 to 17 years of age.
The Pfizer-BioNTech Covid-19 Vaccine is administered as a series of two doses, three weeks apart, the same dosage and dosing regimen for 16 years of age and older.
All three makers of the authorized Covid-19 vaccines—Pfizer, Moderna and Johnson & Johnson—are conducting research on the safety and effectiveness of their vaccines in children in ages as young as six-months old.
Approximately 29 percent of American Indians and Alaska Natives are under the age of 18 while 21.9 of the total United States population is under the age of 18, according to the U.S. Census Bureau.
More Stories Like This
New Mexico Will Investigate Forced Sterilization of Native American WomenUSDA Expands Aid for Lost Farming Revenue Due to 2025 Policies
Two Feathers Native American Family Services Wins 2026 Irvine Leadership Award
Bill Would Give Federal Marshals Authority to Help Tribes Find Missing Children
Indian Health Service to Phase Out Mercury-Containing Dental Amalgam by 2027