
- Details
- By Levi Rickert
WASHINGTON — The U.S. Food and Drug Administration (FDA) on Friday night issued an Emergency Use Authorization (EUA) for the Pfizer-BioNTech vaccine for the prevention of 2019 coronavirus disease (COVID-19) for individuals 16 years of age and older caused by SARS-CoV-2.
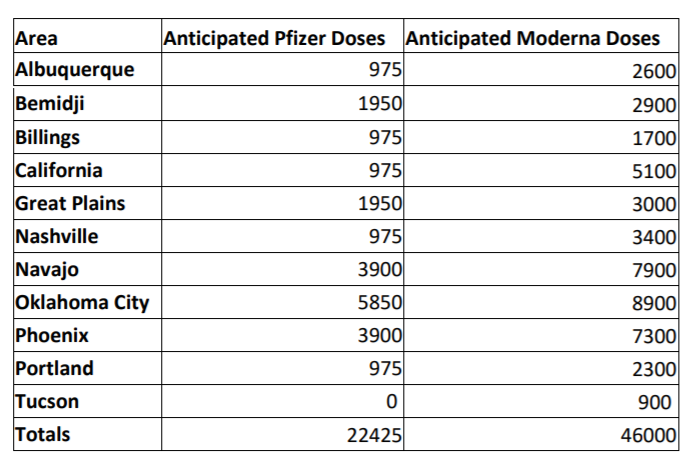
This EUA represents the first vaccine authorized for the prevention of COVID-19. The Pfizer vaccine will arrive in Indian Country this coming week.
More Stories Like This
Native News Weekly (August 25, 2024): D.C. BriefsNavajo Nation Mourns the Passing of Former Vice President Rex Lee Jim
Deb Haaland Earns Endorsement From Communications Workers of America Local 7076
University Soccer Standout Leads by Example
Two Native Americans Named to Democratic Congressional Campaign Committee's“Red to Blue” Program
Help us defend tribal sovereignty.
At Native News Online, our mission is rooted in telling the stories that strengthen sovereignty and uplift Indigenous voices — not just at year’s end, but every single day.
Because of your generosity last year, we were able to keep our reporters on the ground in tribal communities, at national gatherings and in the halls of Congress — covering the issues that matter most to Indian Country: sovereignty, culture, education, health and economic opportunity.
That support sustained us through a tough year in 2025. Now, as we look to the year ahead, we need your help right now to ensure warrior journalism remains strong — reporting that defends tribal sovereignty, amplifies Native truth, and holds power accountable.
 The stakes couldn't be higher. Your support keeps Native voices heard, Native stories told and Native sovereignty defended.
The stakes couldn't be higher. Your support keeps Native voices heard, Native stories told and Native sovereignty defended.
Stand with Warrior Journalism today.
Levi Rickert (Potawatomi), Editor & Publisher

