- Details
- By Native News Online Staff
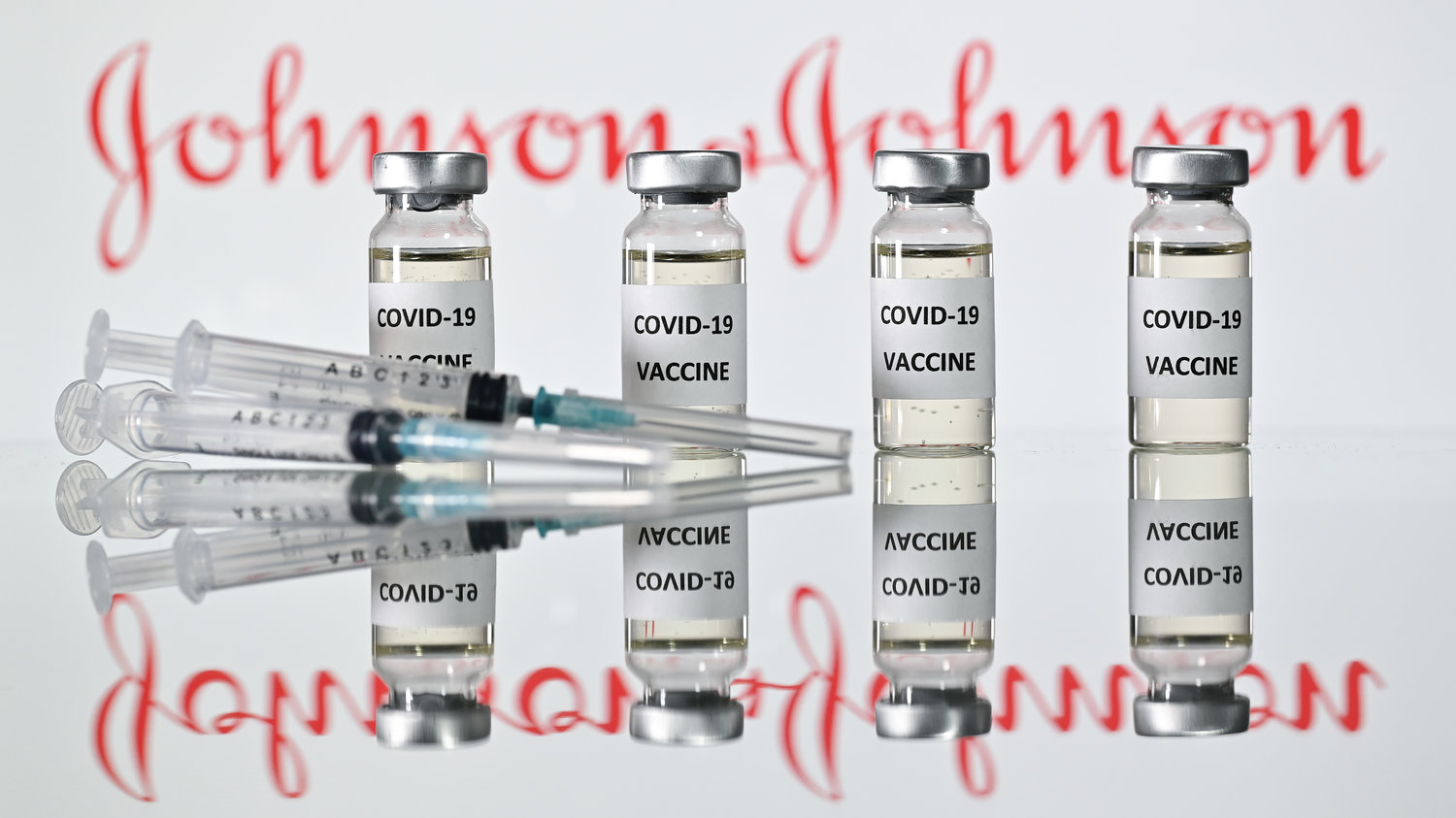
WINDOW ROCK, Ariz. — The Navajo Nation on Tuesday paused the use of the Johnson & Johnson Covid-19 vaccine after the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) recommendation was announced.
Earlier Tuesday, both federal health agencies recommended the pause in the use of the vaccine based on six reported U.S. cases, out of 6.8 million doses administered nationally, of a rare and severe type of blood clot in individuals after receiving the Johnson & Johnson vaccine.
“We fully support pausing use of the Johnson & Johnson vaccine on the Navajo Nation until we receive the findings of the investigation. Navajo Area IHS informed us that approximately 4,000 doses of the Johnson & Johnson had been administered on the Navajo Nation,” Navajo Nation President Jonathan Nez said.
Nez said there have been no major side effects reported on the nation’s largest Indian reservation.
“We will continue working with the Navajo Department of Health, Navajo Area IHS, and tribal health facilities to monitor the status of those who received this particular vaccine. Our health care experts indicate that Tuesday’s announcement by the CDC and FDA does not impact the Pfizer and Moderna vaccines,” Nez continued.
According to the CDC, the individuals were all women between the ages of 18 and 48 and symptoms occurred 6 to 13 days after being vaccinated. The events reported are extremely rare and further findings from the CDC and FDA review with Johnson & Johnson is forthcoming.
According to an IHS statement issued on Tuesday evening, COVID-19 vaccine safety is a top priority for the federal government and is taking all reports of adverse events seriously and has vaccine safety monitoring systems in place. To date, there have been no cases reported through IHS of the rare and severe type of blood clot seen in some individuals who have received the Johnson & Johnson/Janssen vaccine.
As a precaution, if you received the Johnson & Johnson vaccine, and experience severe headache, abdominal pain, leg pain, or shortness of breath within the three weeks after receiving the vaccine, please contact your health provider immediately. People should not be concerned about mild headaches and body aches in the first few days after vaccination. Those are common, temporary side effects brought on by the immune system’s response to the vaccine.
More Stories Like This
Diné-led nonprofit immerses children in their native languageThe mystery of wildlife and a world beyond our understanding
Feds close to releasing draft environmental review of Colorado River management options
Happy Holidays from Native News Online
After Trump cuts, seeds sit in the warehouse
Help us defend tribal sovereignty.
At Native News Online, our mission is rooted in telling the stories that strengthen sovereignty and uplift Indigenous voices — not just at year’s end, but every single day.
Because of your generosity last year, we were able to keep our reporters on the ground in tribal communities, at national gatherings and in the halls of Congress — covering the issues that matter most to Indian Country: sovereignty, culture, education, health and economic opportunity.
That support sustained us through a tough year in 2025. Now, as we look to the year ahead, we need your help right now to ensure warrior journalism remains strong — reporting that defends tribal sovereignty, amplifies Native truth, and holds power accountable.
 The stakes couldn't be higher. Your support keeps Native voices heard, Native stories told and Native sovereignty defended.
The stakes couldn't be higher. Your support keeps Native voices heard, Native stories told and Native sovereignty defended.
Stand with Warrior Journalism today.
Levi Rickert (Potawatomi), Editor & Publisher